Stabilizing the formula – How to choose an emulsifier?
DETERMINATION OF THE CHEMICAL TYPE
As stated, the characterization of the most suitable chemical type is as important as the determination of the required HLB value. For example, an ethoxylated oleic ester of sorbitan gives the oil phase its unsaturated lipophile part. This type of chain exerts an attraction for oils with unsaturated chains. On the other hand, an ethoxylated stearic ester of sorbitan with an analogous HLB value gives the oil phase its saturated lipophile part, undoubtedly more attractive to oils having saturated chains. The emulsifier that shows most affinity with the components of the oil phase will therefore be the most effective. Also, in this case a systematic experimental process would supply the answers.
Let us suppose for example that a required HLB value of twelve has been determined. It will be necessary to try only one mixture of emulsifiers for every chemical class to identify the one capable of supplying the characteristics we want. In fact, it can be shown that in the case being considered, emulsifiers based on lauric acid provide a better control of the viscosity and a satisfactory stability also at low concentrations with respect to emulsifiers based on oleic or stearic acid.
If the emulsifier system is made up of products with different chemical structures, it should be taken into account that the HLB values obtained from the calculation. It is therefore advisable to work between values of ±1.
THE INFLUENCE OF THE HLB VALUE AND THE CONCENTRATION OF THE EMULSIFIER ON THE STABILITY OF THE EMULSION.
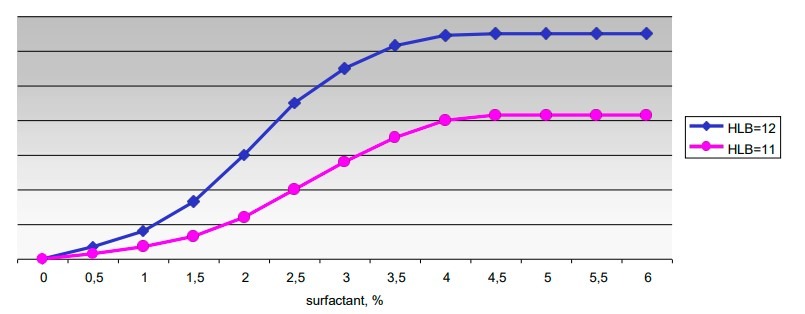
Let us suppose it has been found that a value of HLB = 12 is required to emulsify a given oil. Using emulsifiers of the same chemical class, but with a HLB of 11 and comparing the results with those obtained using emulsifiers having the exact HLB required value, the following graph is obtained.
Here it appears that for concentrations of emulsifier equal to zero the stability of the emulsion is also zero, that at a concentration of 3% the emulsion with a value HLB = 12 is stable and that the stability does not increase with an increase in the concentration of the emulsifier.
THE INFLUENCE OF THE HLB VALUE AND THE CHEMICAL NATURE OF THE EMULSIFIER ON THE STABILITY OF THE EMULSION.
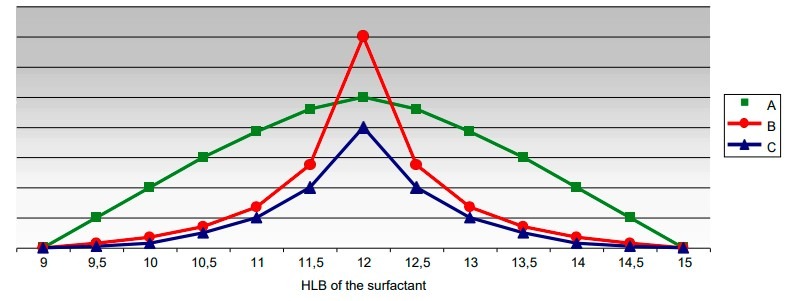
Now let us try to look at the behavior of three emulsifier systems having a HLB = 12 but belonging to different chemical classes. The results obtained with the emulsifier system A, B and C are shown in the following graph. All three of the emulsifiers belonging to the three different chemical classes produce emulsions with the greatest stability around the value HLB = 12 but B gives the best result.
With the A and C systems poorer results are obtained.
These can be improved by increasing the emulsifier concentration without, however, achieving the same values as B.
Therefore, not all the emulsifiers that produce a suitable HLB value will give the same result when
emulsifying a given oil phase. The different behavior of one emulsifier when compared with another is
due to the chemical class it belongs to; this can be exploited to obtain specific results. For example, the
emulsifier system A is more suitable than B if an emulsion with stability over a larger HLB range is
required, even if the peak stability is lower.
