HLB TEMPERATURE and HLB NUMBER – How to choose an emulsifier?
In a nonionic surfactant solution, the effect of increasing the temperature is equivalent to shortening the ethylene oxide chain; a variation in temperature corresponds therefore to a change of the HLB (but not of the HLB number). So a temperature can be determined at which the HLB of a nonionic surfactant is balanced (HLB temperature) and this value coincides with the PIT. The correlation between the “HLB number” and the “HLB temperature” is linear for the ethoxylated dodecyl alcohols. Not all surfactants and not all types of oil give a linear correlation, but it has been confirmed that there is a tendency for high values of HLB number to correspond with high inversion temperatures and vice versa.
INVERSION TEMPERATURE OF EMULSIFIER SYSTEMS
As described before a difference in behavior and stability is often observed in emulsions prepared with emulsifiers belonging to different chemical classes, even if they are combined in such a way as to give the same “HLB number”. An aid to understanding this phenomenon could come from the inversion temperature which being a real measure of the conditions at which the balance of the hydrophile – lipophile properties of the emulsifier occurs could give a more reliable evaluation of the HLB of the system. For example, let us look at the change in behavior of a sorbitan monooleate (SMO) series at different degrees of ethoxylation with change in the relationship between the HLB number and the PIT. The system used is a water/oil system of mineral oil/SMO(n)EO the O/W ratio by weight is 1:1 and the concentration of the surfactant system is 2% of the total.
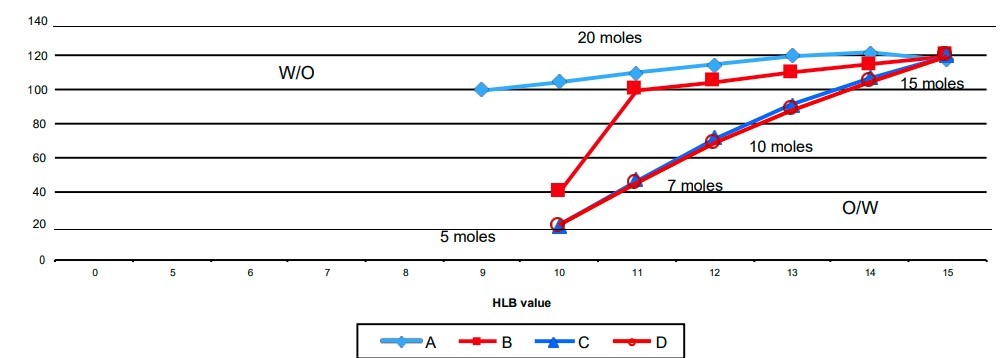
Curve D has been drawn following the SMO’sat varying degrees of ethoxylation, without mixing them. In this case the N(HLB)/PIT relationship is very nearly linear. Curve C was produced with mixtures of various composition of SMO (5 EO) and SMO(20 EO). The behavior of these mixtures is very close to that of the pure sorbitan monooleate mixtures. On the other hand the curves B and A were produced with the pairs SMO(2 EO)/SMO (20 EO) and SMO/SMO (20 EO)respectively.
The bigger the difference in HLB between the two components of the emulsifier couple the greater the deviation from linearity of the N(HLB)/PIT relationship. In the extreme case of a SMO and SMO( 20 EO) couple it is not possible to bring the PIT into the range of temperatures between 0°C and 100°C since further additions of SMO produce a reduction of the PIT to below 0°C. For a mixture of SMO(5 EO) and SMO(20 EO) it is possible to vary continuously the hydrophile-lipophile properties of the emulsifier system by changing the ratio of the two components in the mix. However, it is not possible to do the same with a mixture made of SMO and SMO(20 EO) in which the hydrophile-lipophile properties remain almost constant with variation of the HLB number but then change abruptly on increasing the percentage of the second component.
STORAGE AND STABILITY TEMPERATURE OF EMULSIONS
The average diameter of the internal phase droplets is an indication of the stability of the emulsions: the faster the increase of diameter with time the more rapidly the coagulation and separation processes occur.
In an oil in water emulsion the average diameter of the oil droplets is a function of the emulsification temperature. The nearer it is to the PIT the smaller the average diameter of the droplets becomes. If the emulsion is kept at a storage temperature that is close to the PIT the coagulation of the system happens more quickly compared with those kept at lower storage temperatures. If the emulsification and storage occur at the same temperature at a range between 10 -20°C inclusive, below the PIT, the separation of the aqueous phase will be minimized and therefore the stability will be maximized. To exploit this particular property more fully, emulsions should be produced at a temperature close to the PIT (2-4°C below is better) and then rapidly cooled to the storage temperature in order to slow down the coagulation of the oil droplets and stabilize the final dispersion obtained. In fact, emulsification at a slightly lower temperature than the inversion temperature enables a very fine dispersion of the oil phase to be obtained. This is due to the low interfacial tension and to the formation of a third phase called the surfactant phase which forms very close to the PIT.
This technique showed that the maximum stability is obtained at a storage temperature 25 – 75°C lower than the PIT. The phase inversion temperature provides a less approximate indication than the HLB of a nonionic emulsifier. It also allows an interpretation to be made of the different effects of the temperature of emulsification or the storage and the type of emulsifier system used and gives a rational explanation for some of the phenomena underlying the stability of emulsions. The production process can also benefit A B C D 1 from PIT studies as, when working appropriately, it is possible to obtain fine dispersions spontaneously without needing a high mechanical energy contribution.
