Biodegradability – Introduction – COLORMULS Xtreme
Test Substance
Specification
The following information of the test substance was provided by the sponsor.
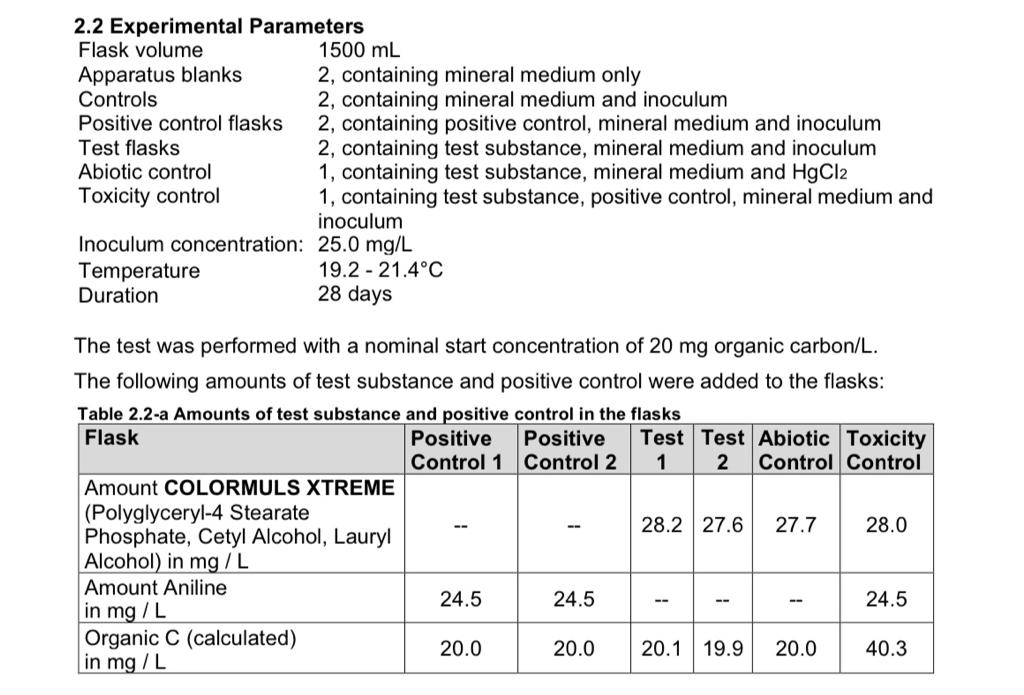
Name COLORMULS XTREME (Polyglyceryl-4 Stearate Phosphate, Cetyl Alcohol, Lauryl Alcohol)
Batch no. not stated
Expiry date not stated
Storage not stated
Pre-Treatment
The carbon content of 72.5 % was determined by elemental analysis. The test item was used directly, in amounts according to its carbon content.
Positive Control
Aniline (Phenylamine, C6H5NH2, CAS-No. 62-53-3) was used as readily bio-degradable positive control. A stock solution containing 2127.8 mg/L in deionized water was prepared and its organic carbon content was measured with 1729.9 ppm, corresponding to an organic carbon content of the positive control of 81.4 %
Test System
Specification
Activated sludge from a biologic sewage treatment plant was used.
The chosen plant is treating mostly domestic sewage.
Source and Pre-Treatment
Source
The sludge was taken from the activation basin of the ESN (Stadtentsorgung Neustadt) sewage treatment plant, Im Altenschemel, NW-Lachen-Speyerdorf.
Date of collection: 09 August 2020, batch no: 20150327.
Pre-Treatment
The sludge was filtrated, washed with tap water twice, then washed with and re-suspended in test medium. It was then aerated for > 12 hours.
The dry matter was determined with 4880 mg suspended solids/litre.
Chemicals
All chemicals used in the test were “analytical grade” or otherwise proved suitable.
Stock solution

Sodium Hydroxide
NaOH, 0.25-m solution, used for trapping of emitted carbon dioxide. NaOH, 1.5-m solution, used for scrubbing of purified air.
Mercury Chloride
HgCl2, used for poisoning of abiotic flasks
Barium hydroxide
Ba(OH)2 solution, used for checking the purified air (saturated solution ,1:3 diluted).
Hydrochloric Acid
HCl, 2-m solution, used for driving off dissolved CO2 on day 28.
Reference Items for Carbon Determination
Potassium hydrogen phthalate for TC, Na2CO3 and NaHCO3 for IC.
Test Vessels
All glassware was cleaned with the laboratory cleaning agent and then rinsed with tap water (thrice), diluted HCL (once), tap water (thrice) and deionized water (thrice).
2000 mL-SCHOTT-flasks were used as test vessels, 100 mL scrubber flasks as absorbent vessels.
PERFORMANCE OF THE STUDY
Preparations
The medium was prepared from the stock solutions. The inoculum was taken from its source, washed, aerated and the dry matter was determined. The test vessels were filled with medium and inoculum.
Then all flasks were aerated for 72 hours with purified, CO2-free, moistened air to purge the system of CO2.
Apparatus
The test vessels were aerated with purified (by activated charcoal), CO2 -scrubbed, moistened air.
The scrubbing of carbon dioxide was achieved by bubbling the purified air through a flask containing 1.5 m-NaOH. To control the absence of CO2, the air was then led through a flask containing a solution of Ba(OH)2 before reaching the test vessels.
Magnetic stirrers were used to prevent deposition of inoculum. The emitted CO2 was trapped in 0.25-m-NaOH. Two scrubbers containing 100 mL each were
connected in series to the test vessels. The initial IC value of the 0.25 m-NaOH was separately determined in each flask.
Sampling
From each front scrubber flask, ten samples were taken in order to determine the emitted CO2. (on days 0, 2, 4, 7, 9, 11, 14, 18, 23 and 29). The sample volume was 1 mL.
The resulting change in the volume of the front flask was considered in the calculation of emitted CO2.
On day 28, 5 mL HCl 2-m. were added to each test flask in order to drive off dissolved CO2.
On day 29, samples from both scrubber flasks were taken.
CO2 Determination
Analyses of the emitted CO2 were made by IC measurement using the carbon analyzer TOC multi N/C 2100S, Analytic Jena. Each sample was measured at least in duplicate.
The carbon
analyzer was calibrated with freshly prepared reference solutions containing potassium hydrogen phthalate and sodium carbonate once a week.
After every start, quality control samples were measured.
